About
Biologists have long suspected sponges (phylum Porifera) were one of the first multicellular organisms on the planet. According to researchers at the world-famous Massachusetts Institute of Technology (MIT), sponges pre-date the Cambrian explosion, an evolutionary period that began around 540 million years ago, during which many new animals appeared.
Sponges are are sessile, meaning that they live their entire adult life attached to a single spot. They do not possess true tissue, but have different cell types with different functions, which together carry out normal body functions. Sponges obtain food and oxygen by filtering large amounts of surrounding water. In addition, many sponge types exist through symbiosis with micro-organisms. Archaea, bacteria, fungi, cyanobacteria, and microalgae can all co-exist with sponges. While sponges are not naturally photosynthetic, they often gain photosynthetic nutrients through a symbiotic relationship with chlorophyll based organisms. Because of sponge inability to move around, over millennia they have evolved a unique ability to produce chemicals to defend themselves from certain dangers in their environment including diseases, other animals and extreme environmental conditions. These chemicals coupled with their unique physical properties that make sponges valuable sources of pharmaceutical compounds.
Drugs from the Sea
IntroductionSponge-Derived Secondary Metabolites with Pharmaceutical Potential
The success rate of finding a new active chemical is 500 times higher in marine organisms than from terrestrial sources.
Mariane sponges have proven to be the most prolific source of novel therapeutic agents, with biomedical action superior to that of existing pharmaceuticals. They have held promise for the development of new medicines since the discovery of antiviral and antileukaemic sponge nucleosides by Bergmann and Feeney in the 1950’s (Bergmann and Feeney, 1950, 1951). Since then, thousands of sponge-derived bioactive compounds have been discovered (Faulkner 2000, 2001, 2002; MarinLit, 1999) increasing the importance of sponges as a high potential source of new drugs. Sponges have the potential to provide future drugs against important diseases, such as cancer, a range of viral diseases, and inflammations.
Over 18,000 marine natural products have been described. Over 30% of those products are derived from sponges. Of the antitumor natural product patent registrations in recent years, over 75% are from sponges1,2. The biological effects of newly discovered metabolites from sponges have been reported in hundreds of scientific papers. Although the molecular mode of action of most metabolites is still unclear, for a substantial number of compounds, the mechanisms by which they interfere with the pathogenesis of a wide range of diseases have been reported. This knowledge is one of the key factors necessary to transform bioactive compounds into medicines. Sponges produce a plethora of chemical compounds with widely varying carbon skeletons, which have been found to interfere with pathogenesis at many different points. The fact that a particular disease can be fought at different points increases the chance of developing selective drugs for specific targets.
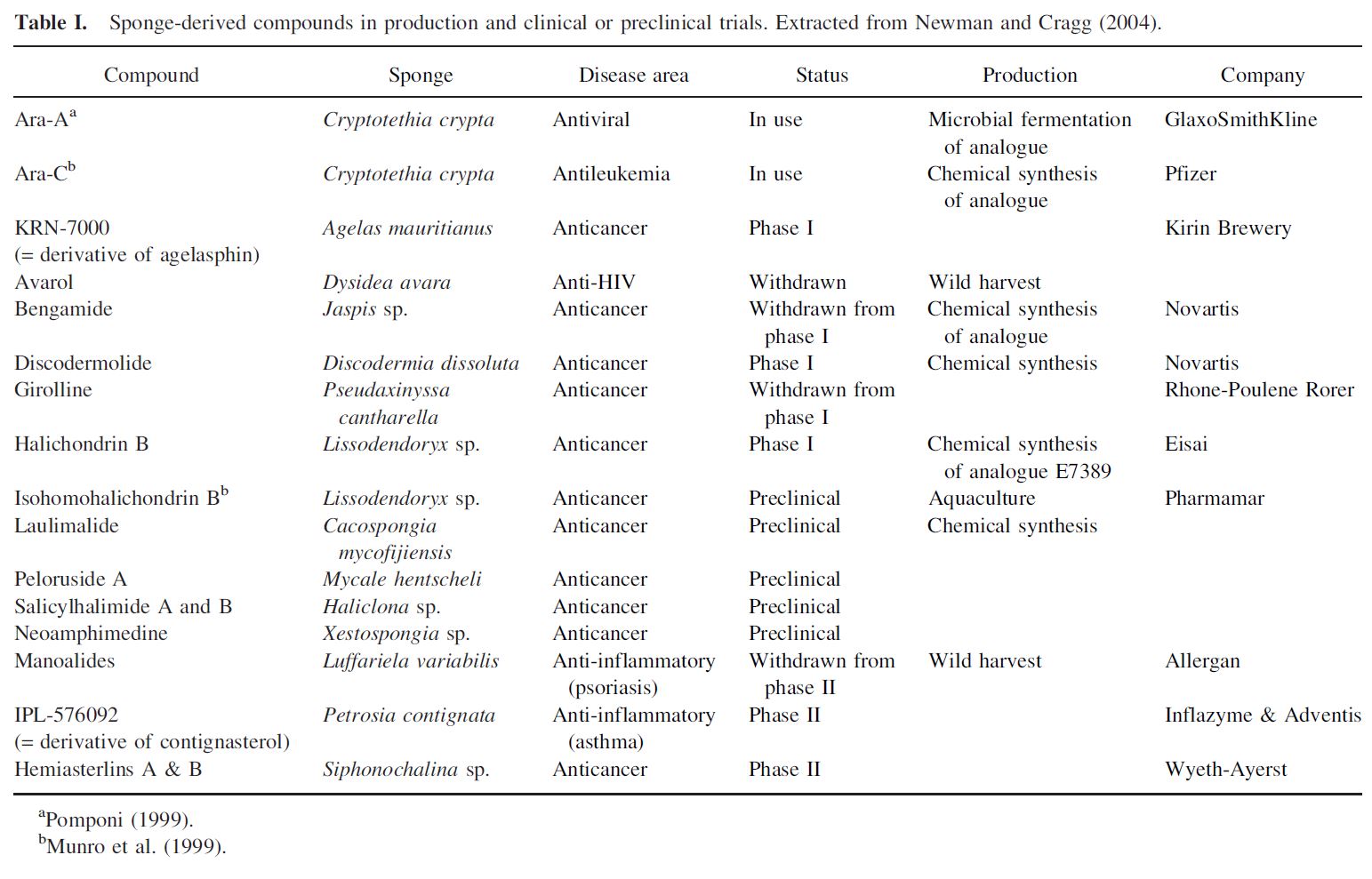
A number of products have entered preclinical and clinical trials (Table I). Ara-A and Ara-C (derivatives of the sponge nucleosides discovered by Bergmann and Feeney) have advanced to the pharmaceutical market as antiviral and antileukemic agents, respectively (McConnell et al., 1994).
1. [Faulkner DJ. Marine natural products. Nat Prod Rep. 2002;19:1–48. [PubMed]]↩
2. [Sipkema D, Franssen MCR, Osinga R, Tramper J, Wijffels RH. Marine sponges as pharmacy. Mar Biotechnol. 2005;7:142–162. [PubMed]]↩
Supply Challenge other companies face
Why aren't there more sponge-dervived drugs on the Market?
If it were only a matter of bioactivity, or having a unique pharmacological profile, hundreds of sponge-derived compounds could be expected to enter drug development in the coming decades. However, bioactivity alone does not determine whether or not a compound enters drug development in the pharmaceutical industry. Other factors such as establishment of a cost-effective source of large-scale supply, and ‘‘drugability’’ in a human model system are paramount in determining if a pharmaceutical company will prioritize a new natural product discovery for inclusion in its drug development pipeline. Expensive to produce, or scarcely available metabolites will only have a chance of entering drug development if they have truly unique pharmacological profiles including new mechanisms of action against a particular drug target or disease. Looking back on those sponge-derived compounds that have advanced to human clinical testing, it can be seen that all were found to be amenable to cost-effective chemical synthesis, thus solving the supply question early-on in the development continuum (Table I).
Wild harvest
Wild harvest of a prolific sponge that is the source of a desired metabolite would be an easy way to acquire a compound for preclinical studies and possibly even early-stage clinical trials. One only needs SCUBA diving equipment and a small boat for a first harvest, thus foregoing the need for investing in and setting-up complex culture systems for an organism that has been notoriously difficult to culture (Osinga et al., 1999).
However, while the wild harvest of marine invertebrates is considered plausible for pre-clinical studies, the collection of larger populations for clinical development and commercial production of an eventual clinical candidate is environmentally unsustainable due to insufficient or inaccessible natural populations and the typical low yields of bioactives1,2. Even so, halichondrin B pre-clinical development was started by harvesting more than one metric ton of the rare sponge Lyssodendoryx sp. from natural populations to afford only 310 mg of pure compound3. Also, bryostatin 1 progressed to Phase I clinical trials through the wild harvest of nearly 13 tons of the bryozoan Bugula neritina yielding 18 g of the anticancer candidate4.
The Villani Advantage
Sustainable Wild Harvest
Villani is the first company poised to commercialize a sustainable supply of valuable bioactive compounds derived from a wild harvest of aquatic sponge while protecting and maintaining the equilibrium of its ecosystem.
Villani is unique in the biotechnology industry in it's ability to provide highly effective natural bioactive compounds well-suited for pharmaceutical development, and supply the materials in commercial volume without depleting natural resources.
1. [Schmitz, F.J.; Bowden, B.F.; Toth, S.I. Antitumor and cytotoxic compounds from marine organisms. In Marine Biotechnolog. Pharmaceutical and Bioactive Natural Products; Attaway, D.H., Zaborsky, O.R., Eds.; Plenum: New York, NY, USA, 1993; Volume 1, pp. 197–308. [Google Scholar]]↩
2. [Pomponi, S.A. The bioprocess—Technological potential of the sea. J. Biotechnol. 1999, 70, 5–13. [Google Scholar] [CrossRef]]↩
3. [Munro, M.H.G.; Blunt, J.W.; Dumdei, E.J.; Hickford, S.J.H.; Lill, R.E.; Li, S.X.; Battershill, C.N.; Duckworth, A.R. The discovery and development of marine compounds with pharmaceutical potential. J. Biotechnol. 1999, 70, 15–25. [Google Scholar] [CrossRef]]↩
4. [Schaufelberger, D.E.; Koleck, M.P.; Beutler, J.A.; Vatakis, A.M.; Alvarado, A.B.; Andrews, P.; Marzo, L.V.; Muschik, G.M.; Roach, J.; Ross, J.T.; et al. The large-scale isolation of bryostatin 1 from Bugula neritina following good manufacturing practices. J. Nat. Prod. 1991, 54, 1265–1270. [Google Scholar] [CrossRef] [PubMed]]↩